- TOHOKU UNIVERSITY
- Research at Tohoku
University School of Medicine - Tohoku University Hospital
- Tohoku University, Department of Diabetes and Metabolism
- United Centers for Advanced
Research and Translational
Medicine (ART) - Nihon University School of Medicine, Division of Diabetes and Metabolic Diseases
- Iwate Medical University, The Department of Internal Medicine Division of Diabetes and Metabolism
Greetings
I. Introduction
Our laboratory, the Department of Metabolism and Diabetes, was founded by merging the Division of Molecular Metabolism and Diabetes (former professor Yoshitomo Oka) with the Division of Metabolic Diseases (Professor Hideki Katagiri) in May of 2013 at Tohoku University Graduate School of Medicine. We have a clinical branch (Department of Diabetes and Metabolism) at Tohoku University Hospital and have a close association with the Center for Metabolic Diseases, Tohoku University.
The aim of our research is to conquer metabolic diseases such as diabetes, obesity, metabolic syndrome and atherosclerosis. For this purpose, we strive to elucidate the precise mechanisms whereby multi-organ creatures maintain metabolic homeostasis on a whole body level. Blood glucose and body weight, especially, are determined by the sum of metabolic actions in all organs/tissues within the WHOLE BODY. From a physician perspective, our research targets the SYSTEMIC regulation of metabolism.
II. Specific Aims and Research Interests
1. Inter-Organ Neuronal Communication Achieving Metabolic Fine-Tuning
In multi-organ creatures, such as human beings, metabolism in different tissues and organs does not go on independently, but rather in a coordinated and regulated manner throughout the body. This metabolic coordination among many organs requires inter-organ communication systems, which are extremely important for maintaining systemic homeostasis. We have recently discovered several examples of inter-organ metabolic communication via NEURONAL RELAYS consisting of afferent and efferent nerves (Figure). These neuronal relays regulate food intake (Cell Metabolism 2006), energy expenditure (Science 2006) and adaptive thermogenesis (Cell Metabolism 2012).
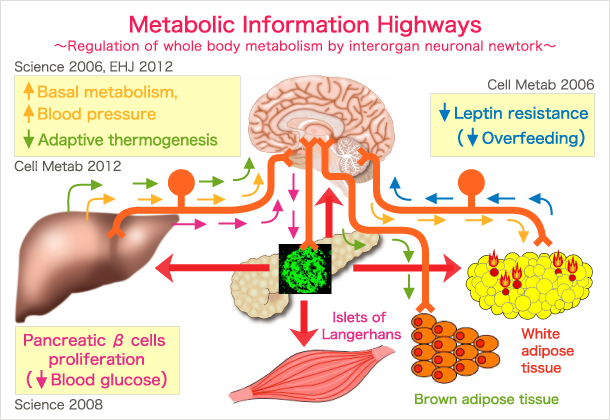
Based on our original findings, we proposed that inter-organ communication via neuronal relays may achieve METABOLIC FINE-TUNING on the whole body level (Circ Res 2007). From the anatomical viewpoint, afferent neuronal signals inevitably pass through and are processed by the brain, leading to coordinated metabolic regulation. Thus, using this system, the brain may function as a conductor coordinating systemic metabolism among many organs which function like an orchestra playing a tune that might be called “METABOLIC HARMONY”. Disharmony may disturb metabolic fine-tuning and cause metabolic disorders such as obesity, diabetes and the metabolic syndrome.
We are now attempting to elucidate the underlying molecular mechanisms in order to apply these endogenous systems to developing novel therapeutic strategies for obesity by manipulating the inter-organ communication systems we have thus far identified. In addition, we are now discovering other inter-organ systems which regulate metabolism on the whole-body level.
2. Inter-Organ Neuronal Communication Achieving Pancreatic β Cell Regeneration
Type 1 diabetes results from selective destruction of pancreatic beta cells. Patients with type 1 diabetes are severely insulin-deficient and must compensate by injecting insulin. Regeneration of insulin-producing cells may become a radical therapy for this disease. Taking advantage of strategies, including bone marrow transplantation, we succeeded in regenerating insulin-producing cells (Endocrinology 2007). In addition, the inter-organ communication system via a neuronal relay achieved pancreatic β cell proliferation (Science 2008). Interleukin-6 also has an impact on potentiating insulin secretion (Diabetes 2009). We are now attempting to elucidate the molecular mechanism involved in our original discoveries. Our highest aspiration is for the research we have conducted to lead to novel regenerative therapies, using the patients’ own cells and systems, which would induce regeneration at the sites where beta cells should be.
3. Vascular Research for Delaying Senescence and Increasing Healthy Life-Spans
Atherosclerosis-derived vascular diseases, such as cardiac infarction and cerebral stroke, are a main cause of death in Japan and other developed countries. First, we endeavored to lower plasma oxidized LDL (oxLDL) by overexpressing lectin-like oxidized low-density lipoprotein receptor 1 (LOX-1), an oxLDL receptor, in the livers of apolipoprotein E knockout mice, a widely used model for atherosclerosis. Intriguingly, hepatic overexpression of LOX-1 transiently decreased plasma oxLDL, without affecting non-oxidized LDL cholesterol levels, and completely inhibited atherosclerotic progression (Circulation 2008).
Downstream from oxLDL, endoplasmic reticulum (ER) stress and inflammatory responses have been implicated in the pathogenesis of arteriosclerosis, such as atherosclerosis and vascular remodeling. We attempted to suppress ER stress responses by creating CHOP deficiency. Using CHOP-deficient mice, CHOP, particularly in vascular cells, was shown to play important roles in accelerating arteriosclerosis (Circulation 2011). We are now manipulating ER stress responses in order to develop therapies for metabolic diseases including atherosclerosis.
In addition, to suppress vascular inflammatory responses, we produced transgenic mice expressing dominant-negative IκB in the endothelium. Inhibition of the nuclear factor-kappa B (NF-κB) pathway by expressing dominant-negative IκB suppressed development of vascular diseases, including atherosclerosis, vascular remodeling and arterial aneurysm formation in specific murine disease models (Cardiovasc Res 2013). Furthermore, endothelial blockade of endothelial NF-κB signaling prevented age-related insulin resistance and vascular senescence and, surprisingly, prolonged lifespans by suppressing senescence (Circulation 2012). Thus, our studies provide insights into the importance of endothelial NF-κB signaling in obesity- and age-related disorders. We are now elucidating the molecular mechanisms whereby endothelial NF-κB is involved in whole body senescence, aiming at developing novel anti-aging strategies.
4. Clinical Studies Designed to Conquer Metabolic Diseases in Humans
In our clinical branch, we currently have several projects underway with the goal of conquering metabolic diseases. For instance, a case with type B insulin resistance syndrome, which is caused by anti-insulin receptor antibodies, was cured by Helicobacter Pylori eradication (Lancet 2009). We are now planning to survey the effectiveness of this therapy for type B insulin resistance syndrome throughout Japan.
In addition, during our clinical activities after the Great East Japan Earthquake, we identified a marker for predicting diabetic patients who would be especially vulnerable after natural disasters (Diabetes Care 2014). Furthermore, we have discovered genome structure abnormalities associated with early onset diabetes (Exp Diabetes Res 2011、PLoS One 2014). These clinical activities have consistently inspired us to conduct research to elucidate the physiological and pathophysiological mechanisms operating in the human body.
Greetings
Research Highlights
- Inter-Organ Neuronal Communication Achieving Metabolic Fine-Tuning
- Inter-Organ Neuronal Communication Achieving Pancreatic β Cell Regeneration
- Vascular Research for Delaying Senescence and Increasing Healthy Life-Spans
- Clinical Studies Designed to Conquer Metabolic Diseases in Humans
- Publication
- Contact us



