- TOHOKU UNIVERSITY
- Research at Tohoku
University School of Medicine - Tohoku University Hospital
- Tohoku University, Department of Diabetes and Metabolism
- United Centers for Advanced
Research and Translational
Medicine (ART) - Nihon University School of Medicine, Division of Diabetes and Metabolic Diseases
- Iwate Medical University, The Department of Internal Medicine Division of Diabetes and Metabolism
研究内容
Inter-Organ Neuronal Communication
Achieving Metabolic Fine-Tuning
Human body consists of more than 270 types of cells, accounting for approximately 60 trillion in number. Various types of cells form up to constitute organs/tissues of the body. Each cell utilizes nutrients such as carbohydrates, fats, and proteins as energy sources, which enable them to function and thus allow their organ/tissue to contribute in maintaining biological activities. Because our body is a closed system, the amount of available energy is always limited and therefore it is crucial that energy metabolism at the whole-body level is tightly regulated so that each organs/tissues and consequently its cells function in a proper way. Inter-organ networks play an essential role in this regulation. Claude Bernard, the author of An Introduction to the Study of Experimental Medicine, once proposed that the regulation of glucose metabolism at the whole-body level is mediated by neural network. Since then, hormones such as insulin and glucagon have been discovered, and the inter-organ network research has dramatically progressed mainly in the field of endocrine system. On the other hand, the significance of inter-organ networks involving autonomic nerves has recently been re-acknowledged owing to elegant series of studies, including those of ours (Fig. 1).
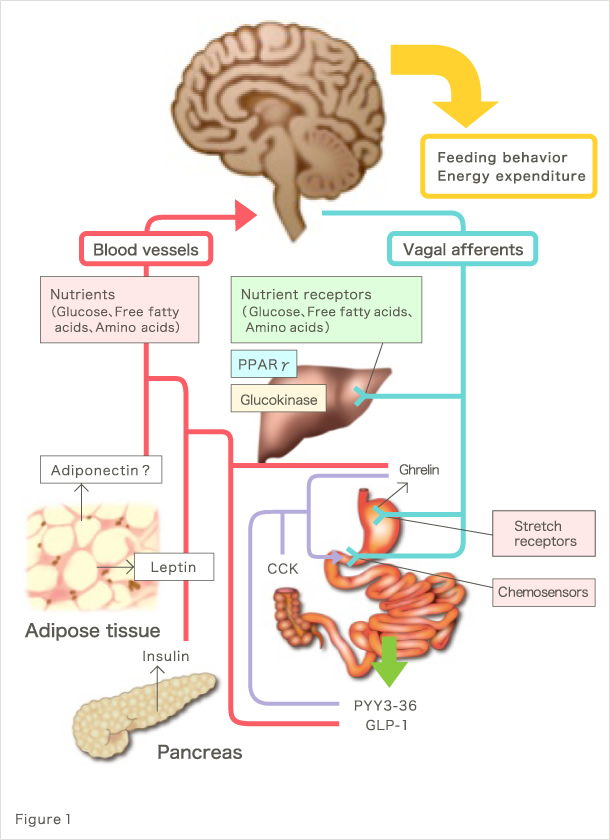
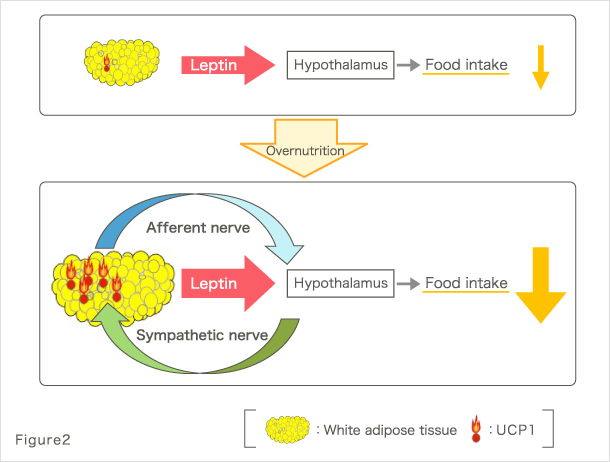
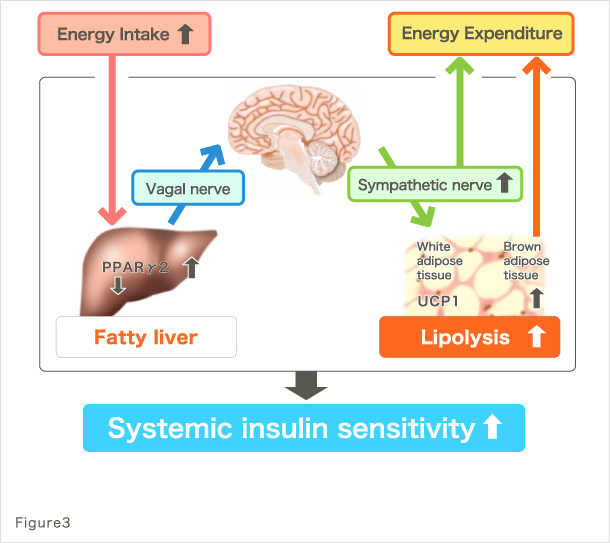
Our research group has been conducting studies toward the understanding of pathologies at the whole-body level in metabolic diseases, including type 2 diabetes and metabolic syndrome. For example, we found that signals emitted by elevated energy consumption in the visceral adipose tissues ameliorate diabetes and suppress appetite through afferent nerve fibers (Fig. 2) (1). These finding were covered in the featured preview on the same issue of Cell Metabolism (2) and in the review of Nature (3), attracting much attention. Furthermore, we found that afferent nerve signals transmitted by hepatic PPARγ during hyperalimentation enhance energy consumption and contribute to the maintenance of weight homeostasis and amelioration of diabetes (Fig. 3) (4). These findings were also covered and attracted much attention in the preview of Cell Metabolism (5). In addition, to the best of our knowledge, we have reported for the first time that the physiological activation of the sympathetic nervous system lowers serum adiponectin levels (6); that diabetes/obesity modulates the biological clock of the central nervous system (7); and that psychological stress modulates the circadian rhythm of hepatic metabolism through the hypothalamus–pituitary–adrenal system (8).
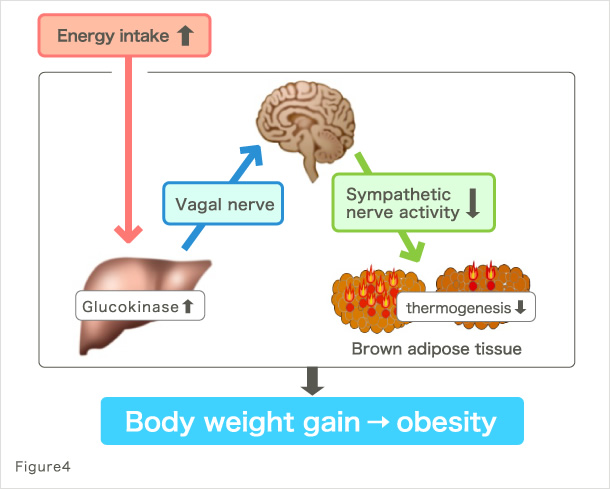
Based on our previous studies, we recently found a new inter-organ neural network controlling the energy storage mechanism at the whole-body level (9). This study revealed an inter-organ neural network from the liver to brown adipose tissue, in which the activation of hepatic glucose metabolism due to hyperalimentation leads to energy storage by suppressing energy consumption in the brown adipose tissue (Fig. 4). Furthermore, our analysis of various sites in the brain revealed the role of brain in this inter-organ neural network and identified a mechanism for the development of obesity. It has previously been considered that the failure in homeostasis mechanism causes weight gain. In our recent study, we demonstrated that the brain controls metabolic information in response to peripheral metabolic changes and actively plays a role in storing energy, leading to body weight gain. Equipping such mechanism must have helped mammals to overcome starvation and conserve themselves (10). Furthermore, these findings have great significance in medicine because they may lead to the elucidation of the basic cause of obesity and answer the question of why we face an explosive increases of obese population in the age of plenty. They also provide insight about fundamental therapeutics or perhaps even prevention of diseases such as diabetes, obesity, and metabolic syndrome.
As mentioned above, the body weight or blood glucose level in multi-organ organisms such as human is not determined by a single organ but is rather regulated by various organs/tissues in the body. As physicians specialized in diabetes and metabolic diseases (11-14), we aim to elucidate the biological phenomena at the whole-body level and contribute to the progress of medical science.
*Review articles: (15), (16), (17), (18)
■ References
- Yamada T*, Katagiri H*, Ishigaki Y*(*co-first author), Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A, Mano H, Aburatani H, Asano T, Oka Y. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 3(3):223-9, 2006.
- Unger, R.H. and J.K. Elmquist, Movin' on up: adipocytes become regulators of nutrient homeostasis. Cell Metab, 3(3): p. 147-8, 2006.
- Rosen, E.D. and B.M. Spiegelman, Adipocytes as regulators of energy balance and glucose homeostasis. Nature, 444(7121): p. 847-53, 2006.
- Uno K*, Katagiri H*, Yamada T*(*co-first author), Ishigaki Y, Ogihara T, Imai J, Hasegawa Y, Gao J, Kaneko K, Iwasaki H, Ishihara H, Sasano H, Inukai K, Mizuguchi H, Asano T, Shiota M, Nakazato M, Oka Y. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 312(5780):1656-9, 2006.
- Schwartz, G.J., Dirty dealing: hepatic vagal afferents reshuffle fat distribution. Cell Metab, 4(2): p. 103-5, 2006.
- Imai J*, Katagiri H*, Yamada T*(*co-first author), Ishigaki Y, Ogihara T, Uno K, Hasegawa Y, Gao J, Ishihara H, Sasano H, Oka Y. Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring). 14(7):1132-41, 2006.
- Kaneko K*, Yamada T* (*co-first author), Tsukita S, Takahashi K, Ishigaki Y, Oka Y, Katagiri H. Obesity alters circadian expressions of molecular clock genes in the brainstem. Brain Res. 1263:58-68, 2009
- Takahashi K, Yamada T (corresponding author), Tsukita S, Kaneko K, Shirai Y, Munakata Y, Ishigaki Y, Imai J, Uno K, Hasegawa Y, Sawada S, Oka Y, Katagiri H. Chronic mild stress alters circadian expressions of molecular clock genes in the liver. Am J Physiol Endocrinol Metab 304(3):E301-9. 2013
- Tsukita S, Yamada T (corresponding author), Uno K, Takahashi1 K, Kaneko K, Ishigaki Y, Imai J, Hasegawa Y, Sawada S, Ishihara H, Oka Y, Katagiri H. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 16(6):825-32. 2012
- Yamada T (corresponding author), Tsukita S, Katagiri H. Identification of a novel inter-organ mechanism favoring energy storage in over-nutrition. Adipocyte. 2(4):281-284. 2013
- Yamada T, Imai J, Ishigaki Y, Hinokio Y, Oka Y, Katagiri H. Possible relevance of HLA-DRB1*0403 haplotype in insulin autoimmune syndrome induced by alpha-lipoic acid, used as a dietary supplement. Diabetes Care. 30(12):e131, 2007
- Ogihara T, Katagiri H, Yamada T, Kudo H, Imai J, Ishigaki Y, Hinokio Y, Yamagiwa Y, Ueno Y, Shimosegawa T, Oka Y. Peginterferon (PEG-IFN) plus ribavirin combination therapy, but neither interferon nor PGE-IFN alone, induced type 1 diabetes in a patient with chronic hepatitis C. Intern Med. 48(16):1387-90, 2009
- Imai J*, Yamada T*(*co-first author), Saito T, Ishigaki Y, Hinokio Y, Oka Y, Katagiri H. Eradication of insulin resistance. Lancet. 374(9685):264, 2009
- Munakata Y, Yamada T (corresponding author), Takahashi K, Tsukita S, Takahashi K, Sawada S, Imai J, Ishigaki Y, Oka Y, Katagiri H. A case of slowly progressive type 1 diabetes with insulin independence maintained for 10 years with α-glucosidase inhibitor monotherapy. Intern Med. 51(24):3391-4.2012
- Yamada T, Katagiri H. Avenues of Communication between the Brain and Tissues/Organs Involved in Energy Homeostasis. (Review) Endocr J. 54(4):497-505, 2007
- Katagiri H, Yamada T, Oka Y. Adiposity and cardiovascular disorders: disturbance of the regulatory system consisting of humoral and neuronal signals. (Review) Circ Res. 101(1):27-39, 2007
- Yamada T, Oka Y, Katagiri H. Inter-organ metabolic communication involved in energy homeostasis: Potential therapeutic targets for obesity and metabolic syndrome. (Review) Pharmacol Ther. 117(1):188-98, 2008
- Yamada T. Inter-organ communications mediate crosstalk between glucose and energy metabolism. (Review) Diabetol Int. 4(3):149-155. 2013
Greetings
Research Highlights
- Inter-Organ Neuronal Communication Achieving Metabolic Fine-Tuning
- Inter-Organ Neuronal Communication Achieving Pancreatic β Cell Regeneration
- Vascular Research for Delaying Senescence and Increasing Healthy Life-Spans
- Clinical Studies Designed to Conquer Metabolic Diseases in Humans
- Publication
- Contact us


