- TOHOKU UNIVERSITY
- Research at Tohoku
University School of Medicine - Tohoku University Hospital
- Tohoku University, Department of Diabetes and Metabolism
- United Centers for Advanced
Research and Translational
Medicine (ART) - Nihon University School of Medicine, Division of Diabetes and Metabolic Diseases
- Iwate Medical University, The Department of Internal Medicine Division of Diabetes and Metabolism
研究内容
Inter-Organ Neuronal Communication
Achieving Pancreatic β Cell Regeneration
Pancreatic β cells play essential roles in the regulation of glucose homeostasis. Therefore, maintenance of β cell mass is a critical issue in developing effective strategies for diabetes treatment. Working from this viewpoint, we previously achieved the production of insulin positive cells in insulin-deficient murine liver in vivo employing an adenoviral gene transduction system [1].
Insulin hypersecretion and pancreatic β cell hyperplasia occur under specific conditions, such as obesity, in response to increasing insulin demand. These compensatory responses of β cells protect individuals against the development of diabetes. However, the mechanism underlying this process is not as yet fully understood. In fact, insulin-resistant animals and human subjects reportedly exhibit hyperinsulinemia and β cell hyperplasia prior to the onset of detectable hyperglycemia, suggesting the existence of an unknown mechanism modulating β cell compensatory responses. We have recently shown inter-organ networks mediated by both neuronal and humoral factors to be involved in this process.
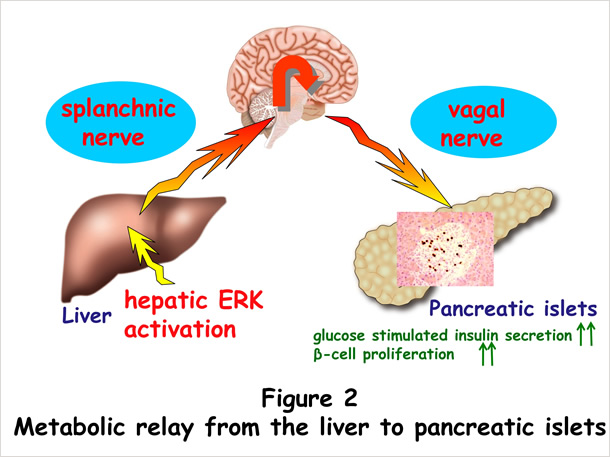
We found that hepatic activation of extracellular signal-regulated kinase (ERK) signaling enhances both insulin secretion and β cell proliferation (Figure 1). We previously identified neuronal signaling as playing important roles in regulating systemic metabolism [2]. Starting with our knowledge of this background, we performed denervation experiments and revealed that these inter-organ effects are mediated by a neural relay from the liver to pancreatic islets. Importantly, blockade of this neural relay in murine obesity models inhibited both insulin secretion and pancreatic islet expansion during obesity development, showing this inter-organ network system to be physiologically involved in compensatory β cell responses. Furthermore, in murine models of insulin-deficient diabetes, hepatic ERK activation normalized blood glucose [3, 4] (Figure 2). We are now studying the molecular mechanism underlying the functions of this system, in order to gain a greater understanding of systemic glucose metabolism regulation and in hopes of developing curative treatments for diabetes.
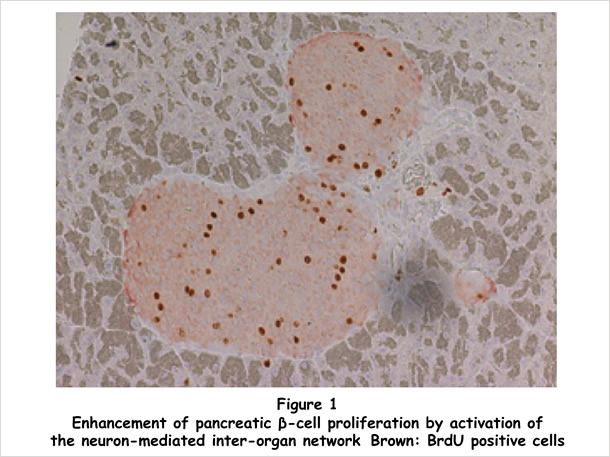
In addition, our results showed that manipulation of endogenous neural machinery can lead to regeneration of a damaged tissue in vivo. We are interested in developing a novel regenerative medicine strategy, based on modulating neural signals, not only for diabetes but also many other degenerative disorders.
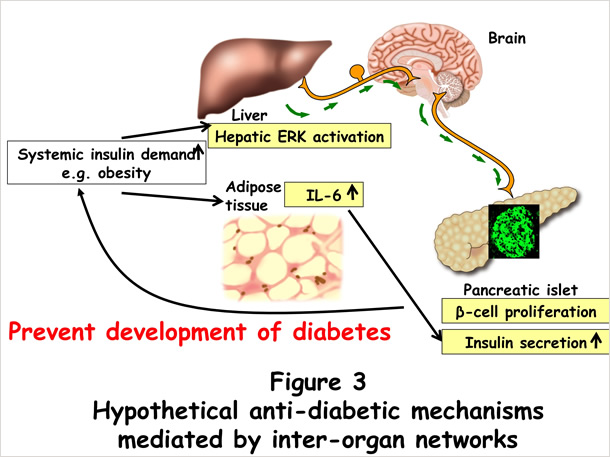
We found that interleukin-6 (IL-6) enhances glucose-stimulated insulin secretion (GSIS) [5]. Since circulating IL-6 levels are known to correlate with fat mass volumes even in the early stage of obesity, IL-6-induced enhancement of GSIS is an inter-organ network, essentially a bridge between adipose tissue and pancreatic islets, which is potentially involved in compensatory hyperinsulinemia during obesity development (Figure 3). We are now exploring other novel inter-organ networks which regulate compensatory pancreatic β cell responses.
We previously reported eradication of Helicobacter Pylori to possibly be a curative treatment for Type B insulin resistance syndrome, a refractory disease characterized by the production of autoantibodies against the insulin receptor [6]. We hope to publish, as physician scientists, further interesting findings from our clinical research and case reports.
■ Reference
- Imai, J., et al., Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem Biophys Res Commun, 2005. 326(2): p. 402-9.
- Imai, J., et al., Cold exposure suppresses serum adiponectin levels through sympathetic nerve activation in mice. Obesity (Silver Spring), 2006. 14(7): p. 1132-41.
- Imai, J., et al., Regulation of Pancreatic {beta} Cell Mass by Neuronal Signals from the Liver. Science, 2008. 322(5905): p. 1250-1254.
- Imai, J., Y. Oka, and H. Katagiri, Identification of a novel mechanism regulating beta-cell mass: neuronal relay from the liver to pancreatic beta-cells. Islets, 2009. 1(1): p. 75-7.
- Suzuki, T., et al., Interleukin-6 enhances glucose-stimulated insulin secretion from pancreatic beta-cells: potential involvement of the PLC-IP3-dependent pathway. Diabetes, 2011. 60(2): p. 537-47.
- Imai, J., et al., Eradication of insulin resistance. Lancet, 2009. 374(9685): p. 264.
Greetings
Research Highlights
- Inter-Organ Neuronal Communication Achieving Metabolic Fine-Tuning
- Inter-Organ Neuronal Communication Achieving Pancreatic β Cell Regeneration
- Vascular Research for Delaying Senescence and Increasing Healthy Life-Spans
- Clinical Studies Designed to Conquer Metabolic Diseases in Humans
- Publication
- Contact us


